COVID-19 EMERGENCY SUPPORT
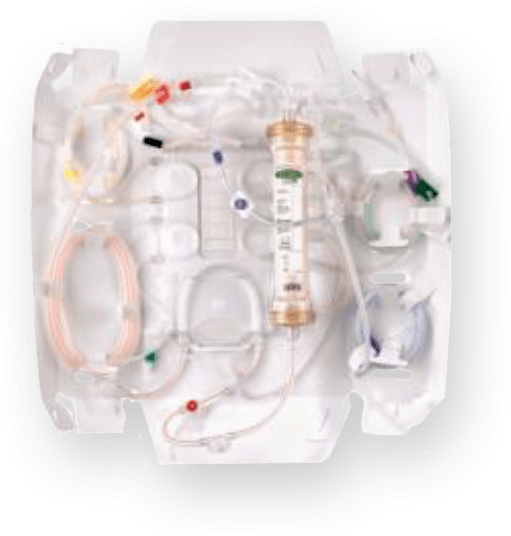
PRISMAFLEX ST FILTER SET
The PRISMAFLEX ST Set has been authorized by the FDA under EUA200704 (Emergency Use Authorization) to provide continuous renal replacement therapy (CRRT) to treat patients in an acute care environment during the Coronavirus Disease 2019 (COVID-19) pandemic. This system is intended for patients who have acute renal failure, fluid overload or both. The device has neither been cleared or approved to provide CRRT in an acute care environment. The device is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of the PRISMAFLEX ST Set under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Like the M-series Filter sets, the PRISMAFLEX ST Filter Set utilizes the proven AN 69 dialyzer membrane. It also contains a polyethyleneimine surface treatment on the membrane, which is intended to reduce local thrombogenesis.